what needs to happen in order for cession in hawaii to occur?
Liquids and Solids
Phase Diagrams
OpenStaxCollege
[latexpage]
Learning Objectives
Past the cease of this section, you lot will be able to:
- Explain the structure and utilize of a typical stage diagram
- Use stage diagrams to identify stable phases at given temperatures and pressures, and to depict stage transitions resulting from changes in these properties
- Draw the supercritical fluid phase of matter
In the previous module, the variation of a liquid'due south equilibrium vapor pressure with temperature was described. Considering the definition of boiling indicate, plots of vapor pressure versus temperature represent how the boiling betoken of the liquid varies with pressure. Also described was the use of heating and cooling curves to determine a substance's melting (or freezing) indicate. Making such measurements over a wide range of pressures yields data that may be presented graphically every bit a phase diagram. A stage diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas stage-transition equilibria of a substance. These diagrams point the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation points, boiling points). A typical phase diagram for a pure substance is shown in [link].
The physical state of a substance and its stage-transition temperatures are represented graphically in a phase diagram.
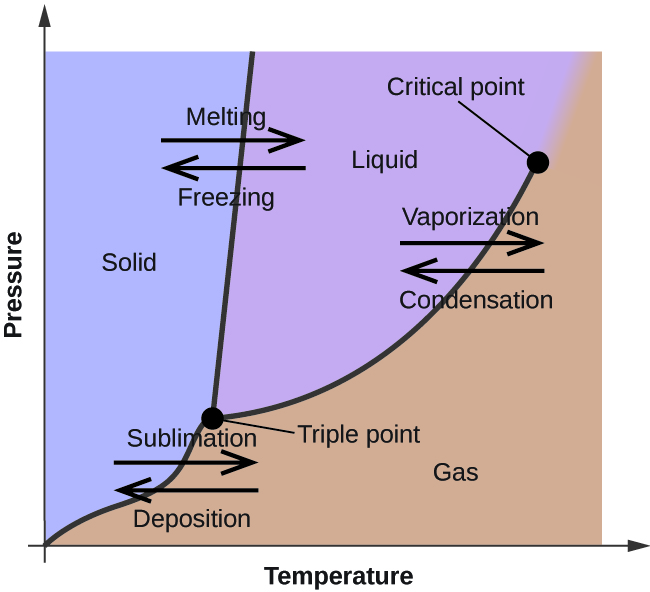
To illustrate the utility of these plots, consider the stage diagram for h2o shown in [link].
The force per unit area and temperature axes on this phase diagram of h2o are not drawn to constant calibration in order to illustrate several important backdrop.
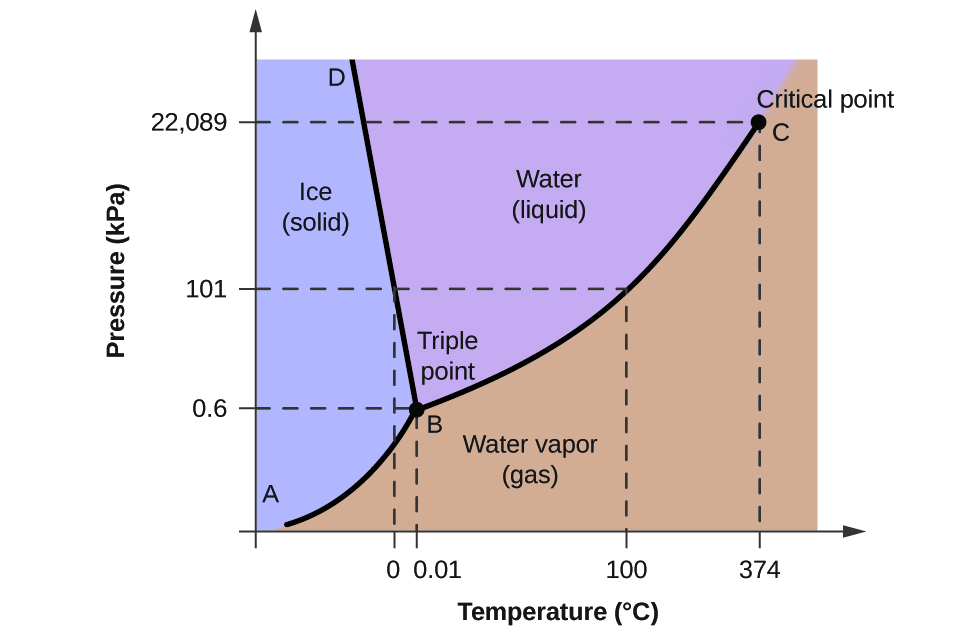
We can apply the stage diagram to place the physical state of a sample of water under specified conditions of pressure level and temperature. For case, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "water ice." Under these conditions, water exists only as a solid (water ice). A pressure of fifty kPa and a temperature of l °C correspond to the "water" region—here, h2o exists only as a liquid. At 25 kPa and 200 °C, h2o exists only in the gaseous country. Notation that on the H2O phase diagram, the force per unit area and temperature axes are non drawn to a constant scale in gild to permit the illustration of several important features as described hither.
The curve BC in [link] is the plot of vapor force per unit area versus temperature every bit described in the previous module of this chapter. This "liquid-vapor" curve separates the liquid and gaseous regions of the phase diagram and provides the humid point for water at whatever pressure. For instance, at 1 atm, the boiling betoken is 100 °C. Notice that the liquid-vapor curve terminates at a temperature of 374 °C and a pressure of 218 atm, indicating that h2o cannot exist as a liquid above this temperature, regardless of the pressure. The physical properties of water under these atmospheric condition are intermediate between those of its liquid and gaseous phases. This unique state of matter is called a supercritical fluid, a topic that will exist described in the next department of this module.
The solid-vapor bend, labeled AB in [link], indicates the temperatures and pressures at which ice and water vapor are in equilibrium. These temperature-pressure data pairs correspond to the sublimation, or deposition, points for h2o. If nosotros could zoom in on the solid-gas line in [link], we would see that water ice has a vapor pressure of about 0.xx kPa at −10 °C. Thus, if we place a frozen sample in a vacuum with a pressure less than 0.xx kPa, ice will sublime. This is the basis for the "freeze-drying" process often used to preserve foods, such equally the water ice cream shown in [link].
Freeze-dried foods, like this ice foam, are dehydrated by sublimation at pressures below the triple bespeak for water. (credit: ʺlwaoʺ/Flickr)

The solid-liquid curve labeled BD shows the temperatures and pressures at which ice and liquid h2o are in equilibrium, representing the melting/freezing points for water. Annotation that this curve exhibits a slight negative slope (greatly exaggerated for clarity), indicating that the melting point for water decreases slightly as pressure increases. Water is an unusual substance in this regard, as most substances exhibit an increase in melting point with increasing pressure. This behavior is partly responsible for the movement of glaciers, like the one shown in [link]. The bottom of a glacier experiences an immense pressure due to its weight that can melt some of the water ice, forming a layer of liquid water on which the glacier may more easily slide.
The immense pressures beneath glaciers effect in partial melting to produce a layer of water that provides lubrication to aid glacial motion. This satellite photo shows the advancing border of the Perito Moreno glacier in Argentina. (credit: NASA)

The point of intersection of all 3 curves is labeled B in [link]. At the pressure and temperature represented by this betoken, all 3 phases of water coexist in equilibrium. This temperature-pressure data pair is chosen the triple point. At pressures lower than the triple point, water cannot exist as a liquid, regardless of the temperature.
Determining the State of Water
Using the phase diagram for water given in [link], determine the state of water at the following temperatures and pressures:
(a) −10 °C and 50 kPa
(b) 25 °C and 90 kPa
(c) 50 °C and 40 kPa
(d) fourscore °C and 5 kPa
(e) −ten °C and 0.3 kPa
(f) 50 °C and 0.3 kPa
Solution
Using the stage diagram for water, nosotros tin can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas.
Check Your Learning
What phase changes can water undergo as the temperature changes if the pressure is held at 0.3 kPa? If the force per unit area is held at l kPa?
Answer:
At 0.3 kPa: \(\text{south}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\dominion{0.2em}{0ex}}\text{g}\) at −58 °C. At 50 kPa: \(\text{s}\phantom{\dominion{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}\text{50}\) at 0 °C, fifty ⟶ thou at 78 °C
Consider the phase diagram for carbon dioxide shown in [link] equally another instance. The solid-liquid curve exhibits a positive slope, indicating that the melting indicate for CO2 increases with pressure equally it does for most substances (water being a notable exception as described previously). Notice that the triple indicate is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid under ambient pressure conditions. Instead, cooling gaseous carbon dioxide at ane atm results in its deposition into the solid state. Likewise, solid carbon dioxide does not melt at one atm pressure but instead sublimes to yield gaseous COtwo. Finally, notice that the critical point for carbon dioxide is observed at a relatively pocket-size temperature and pressure in comparing to water.
The pressure and temperature axes on this stage diagram of carbon dioxide are not drawn to constant scale in order to illustrate several important properties.
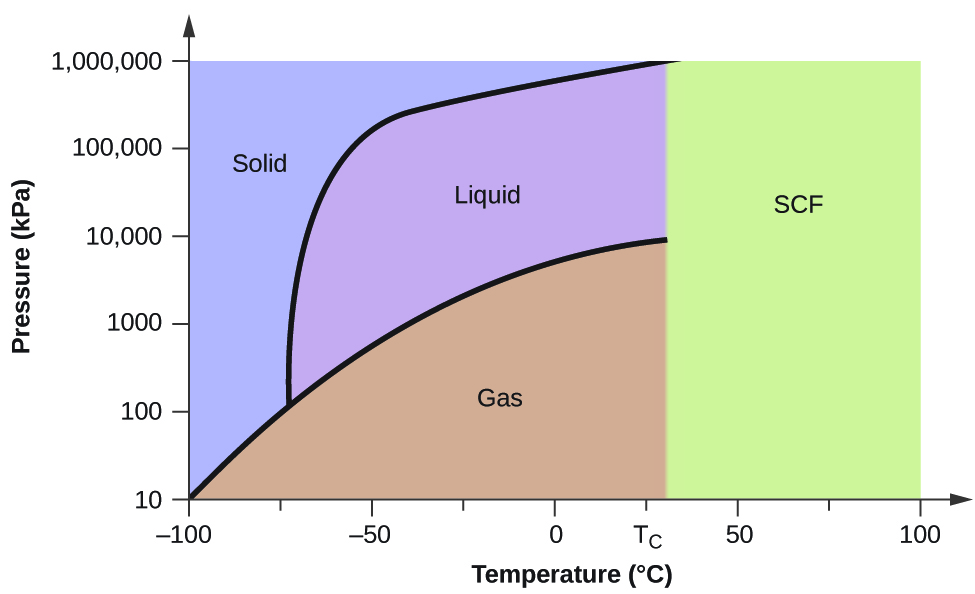
Determining the State of Carbon Dioxide
Using the phase diagram for carbon dioxide shown in [link], determine the land of COtwo at the following temperatures and pressures:
(a) −30 °C and 2000 kPa
(b) −60 °C and k kPa
(c) −60 °C and 100 kPa
(d) twenty °C and 1500 kPa
(e) 0 °C and 100 kPa
(f) 20 °C and 100 kPa
Solution
Using the stage diagram for carbon dioxide provided, we can decide that the land of CO2 at each temperature and pressure given are every bit follows: (a) liquid; (b) solid; (c) gas; (d) liquid; (east) gas; (f) gas.
Bank check Your Learning
Determine the phase changes carbon dioxide undergoes when its temperature is varied, thus holding its pressure constant at 1500 kPa? At 500 kPa? At what approximate temperatures practice these phase changes occur?
Answer:
at 1500 kPa: \(\text{southward}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}\text{50}\) at −45 °C, \(\text{l}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}\text{thou}\) at −10 °C;
at 500 kPa: \(\text{south}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\dominion{0.2em}{0ex}}\text{g}\) at −58 °C
Supercritical Fluids
If we place a sample of water in a sealed container at 25 °C, remove the air, and let the vaporization-condensation equilibrium establish itself, we are left with a mixture of liquid water and water vapor at a pressure of 0.03 atm. A singled-out boundary betwixt the more dense liquid and the less dense gas is clearly observed. As we increment the temperature, the pressure of the water vapor increases, as described by the liquid-gas curve in the phase diagram for water ([link]), and a two-stage equilibrium of liquid and gaseous phases remains. At a temperature of 374 °C, the vapor pressure has risen to 218 atm, and any further increase in temperature results in the disappearance of the boundary between liquid and vapor phases. All of the water in the container is now present in a unmarried phase whose physical backdrop are intermediate between those of the gaseous and liquid states. This phase of matter is called a supercritical fluid, and the temperature and pressure in a higher place which this phase exists is the critical point ([link]). Higher up its critical temperature, a gas cannot be liquefied no matter how much pressure is applied. The pressure required to liquefy a gas at its critical temperature is called the critical force per unit area. The critical temperatures and critical pressures of some common substances are given in [link].
| Substance | Critical Temperature (K) | Critical Pressure (atm) |
|---|---|---|
| hydrogen | 33.2 | 12.8 |
| nitrogen | 126.0 | 33.5 |
| oxygen | 154.three | 49.vii |
| carbon dioxide | 304.ii | 73.0 |
| ammonia | 405.5 | 111.5 |
| sulfur dioxide | 430.3 | 77.7 |
| h2o | 647.1 | 217.seven |
(a) A sealed container of liquid carbon dioxide slightly beneath its critical point is heated, resulting in (b) the formation of the supercritical fluid stage. Cooling the supercritical fluid lowers its temperature and pressure level below the critical point, resulting in the reestablishment of separate liquid and gaseous phases (c and d). Colored floats illustrate differences in density between the liquid, gaseous, and supercritical fluid states. (credit: modification of piece of work by "mrmrobin"/YouTube)
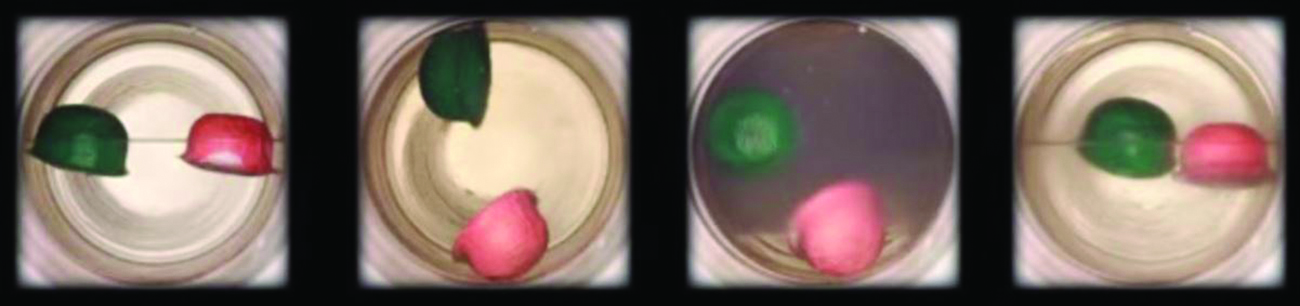
Like a gas, a supercritical fluid will expand and fill a container, but its density is much greater than typical gas densities, typically beingness close to those for liquids. Similar to liquids, these fluids are capable of dissolving nonvolatile solutes. They exhibit essentially no surface tension and very depression viscosities, however, so they can more effectively penetrate very minor openings in a solid mixture and remove soluble components. These properties make supercritical fluids extremely useful solvents for a broad range of applications. For example, supercritical carbon dioxide has get a very popular solvent in the food industry, beingness used to decaffeinate coffee, remove fats from potato fries, and extract flavour and fragrance compounds from citrus oils. It is nontoxic, relatively inexpensive, and not considered to be a pollutant. After utilise, the COtwo tin can be easily recovered by reducing the pressure level and collecting the resulting gas.
The Critical Temperature of Carbon Dioxide
If we shake a carbon dioxide burn extinguisher on a absurd day (18 °C), we tin hear liquid COtwo sloshing effectually inside the cylinder. However, the same cylinder appears to incorporate no liquid on a hot summertime day (35 °C). Explain these observations.
Solution
On the cool day, the temperature of the CO2 is below the critical temperature of CO2, 304 K or 31 °C ([link]), so liquid COii is nowadays in the cylinder. On the hot day, the temperature of the CO2 is greater than its critical temperature of 31 °C. Above this temperature no corporeality of pressure can liquefy CO2 then no liquid COtwo exists in the burn extinguisher.
Check Your Learning
Ammonia can be liquefied by compression at room temperature; oxygen cannot be liquefied under these weather condition. Why exercise the 2 gases exhibit different beliefs?
Answer:
The critical temperature of ammonia is 405.v K, which is higher than room temperature. The disquisitional temperature of oxygen is below room temperature; thus oxygen cannot exist liquefied at room temperature.
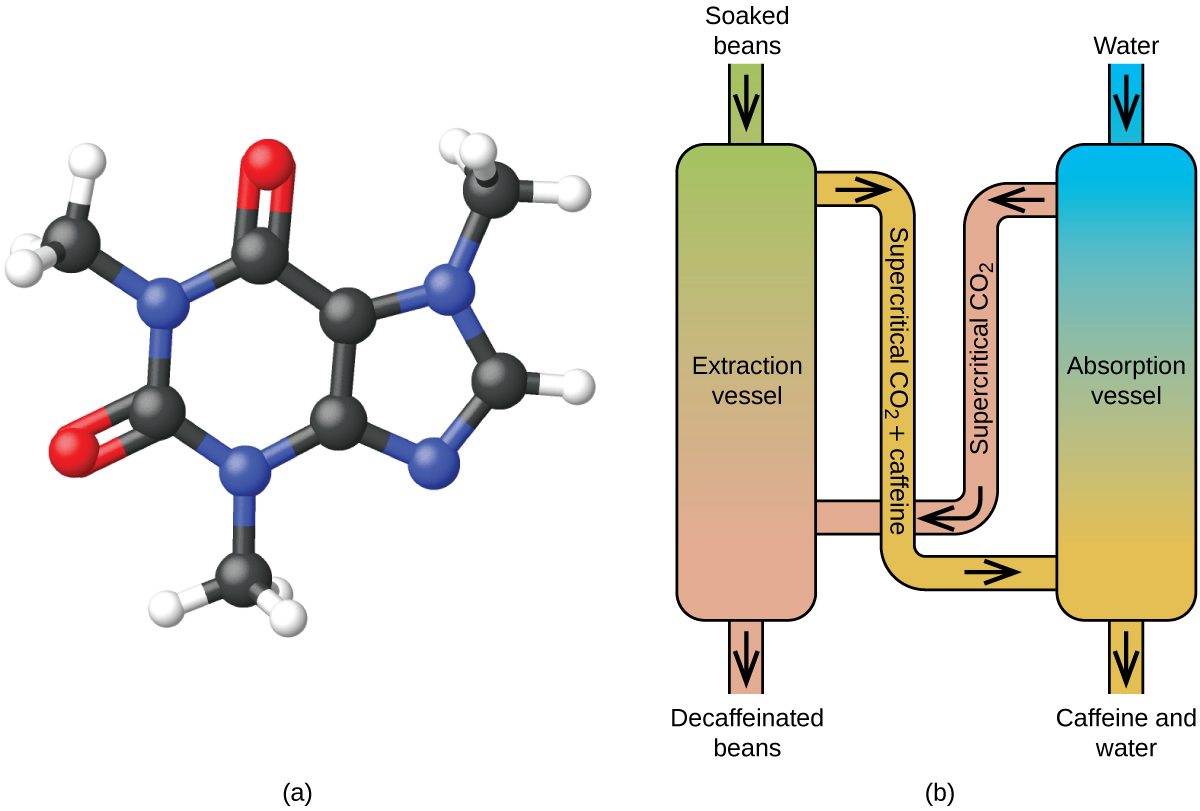
Decaffeinating Coffee Using Supercritical CO2
Coffee is the world'due south 2nd nigh widely traded commodity, following only petroleum. Across the globe, people love coffee'south smell and sense of taste. Many of us also depend on i component of java—caffeine—to aid united states of america go going in the morning or stay warning in the afternoon. But late in the day, coffee's stimulant effect tin keep you lot from sleeping, so you may choose to drink decaffeinated coffee in the evening.
Since the early 1900s, many methods accept been used to decaffeinate coffee. All have advantages and disadvantages, and all depend on the physical and chemical properties of caffeine. Because caffeine is a somewhat polar molecule, it dissolves well in water, a polar liquid. Nevertheless, since many of the other 400-plus compounds that contribute to coffee'due south taste and aroma too dissolve in H2O, hot water decaffeination processes can also remove some of these compounds, adversely affecting the smell and gustation of the decaffeinated java. Dichloromethane (CHiiCl2) and ethyl acetate (CH3CO2C2Hv) take similar polarity to caffeine, and are therefore very effective solvents for caffeine extraction, simply both likewise remove some flavour and olfactory property components, and their employ requires long extraction and cleanup times. Because both of these solvents are toxic, health concerns have been raised regarding the issue of rest solvent remaining in the decaffeinated coffee.
Supercritical fluid extraction using carbon dioxide is now beingness widely used as a more than effective and environmentally friendly decaffeination method ([link]). At temperatures above 304.two K and pressures above 7376 kPa, CO2 is a supercritical fluid, with properties of both gas and liquid. Like a gas, it penetrates deep into the coffee beans; like a liquid, it effectively dissolves certain substances. Supercritical carbon dioxide extraction of steamed coffee beans removes 97−99% of the caffeine, leaving java's flavor and odour compounds intact. Because COii is a gas nether standard conditions, its removal from the extracted coffee beans is hands accomplished, as is the recovery of the caffeine from the extract. The caffeine recovered from java beans via this procedure is a valuable production that can be used later as an additive to other foods or drugs.
(a) Caffeine molecules have both polar and nonpolar regions, making information technology soluble in solvents of varying polarities. (b) The schematic shows a typical decaffeination procedure involving supercritical carbon dioxide.
Central Concepts and Summary
The temperature and pressure level conditions at which a substance exists in solid, liquid, and gaseous states are summarized in a phase diagram for that substance. Phase diagrams are combined plots of iii pressure level-temperature equilibrium curves: solid-liquid, liquid-gas, and solid-gas. These curves represent the relationships between phase-transition temperatures and pressures. The point of intersection of all iii curves represents the substance'southward triple point—the temperature and force per unit area at which all three phases are in equilibrium. At pressures below the triple point, a substance cannot be in the liquid land, regardless of its temperature. The terminus of the liquid-gas curve represents the substance'southward disquisitional point, the pressure and temperature above which a liquid phase cannot exist.
Chemical science Cease of Chapter Exercises
From the stage diagram for water ([link]), decide the country of h2o at:
(a) 35 °C and 85 kPa
(b) −15 °C and twoscore kPa
(c) −15 °C and 0.1 kPa
(d) 75 °C and 3 kPa
(e) 40 °C and 0.ane kPa
(f) threescore °C and 50 kPa
What phase changes will take place when water is subjected to varying pressure at a constant temperature of 0.005 °C? At 40 °C? At −twoscore °C?
At low pressures and 0.005 °C, the h2o is a gas. Every bit the pressure increases to 4.half dozen torr, the h2o becomes a solid; as the pressure increases still more, information technology becomes a liquid. At 40 °C, h2o at low pressure level is a vapor; at pressures college than near 75 torr, information technology converts into a liquid. At −40 °C, h2o goes from a gas to a solid as the pressure increases above very depression values.
Pressure cookers allow food to cook faster because the college pressure inside the pressure cooker increases the boiling temperature of water. A particular pressure level cooker has a safety valve that is prepare to vent steam if the pressure exceeds 3.4 atm. What is the guess maximum temperature that tin be reached within this pressure cooker? Explain your reasoning.
From the phase diagram for carbon dioxide in [link], determine the country of COtwo at:
(a) 20 °C and 1000 kPa
(b) 10 °C and 2000 kPa
(c) ten °C and 100 kPa
(d) −forty °C and 500 kPa
(eastward) −80 °C and 1500 kPa
(f) −80 °C and 10 kPa
(a) liquid; (b) solid; (c) gas; (d) gas; (eastward) gas; (f) gas
Decide the phase changes that carbon dioxide undergoes as the pressure level changes if the temperature is held at −50 °C? If the temperature is held at −twoscore °C? At twenty °C? (See the phase diagram in [link].)
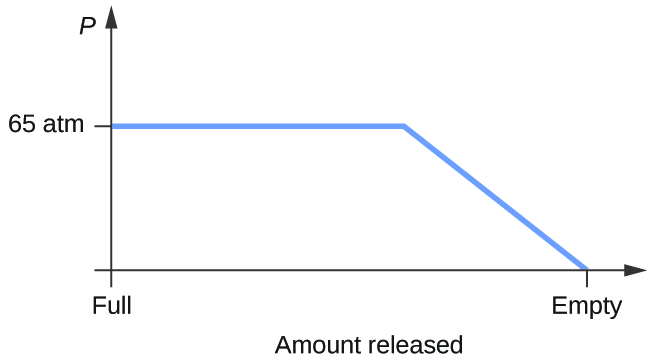
Consider a cylinder containing a mixture of liquid carbon dioxide in equilibrium with gaseous carbon dioxide at an initial force per unit area of 65 atm and a temperature of 20 °C. Sketch a plot depicting the change in the cylinder pressure with time as gaseous carbon dioxide is released at abiding temperature.
Dry out ice, CO2(s), does not cook at atmospheric pressure. It sublimes at a temperature of −78 °C. What is the everyman pressure level at which CO2(south) volition melt to give COtwo(l)? At approximately what temperature will this occur? (Come across [link] for the phase diagram.)
If a severe storm results in the loss of electricity, it may be necessary to use a clothesline to dry laundry. In many parts of the country in the dead of winter, the clothes will quickly freeze when they are hung on the line. If it does not snow, will they dry anyway? Explain your answer.
Aye, ice will sublime, although it may take it several days. Ice has a small vapor pressure level, and some ice molecules grade gas and escape from the ice crystals. As fourth dimension passes, more and more solid converts to gas until eventually the wearing apparel are dry.
Is information technology possible to liquefy nitrogen at room temperature (virtually 25 °C)? Is it possible to liquefy sulfur dioxide at room temperature? Explain your answers.
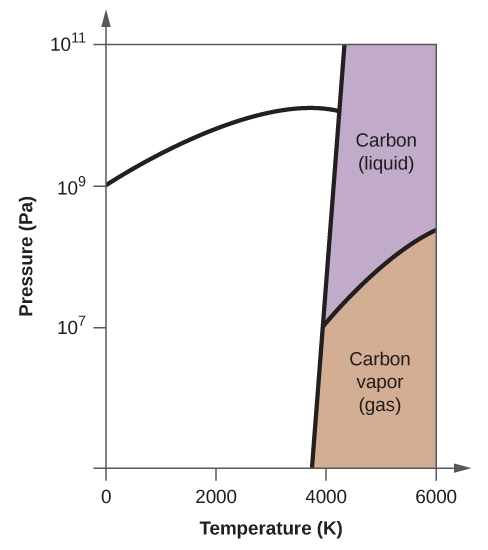
Elemental carbon has one gas phase, i liquid stage, and two different solid phases, equally shown in the phase diagram:
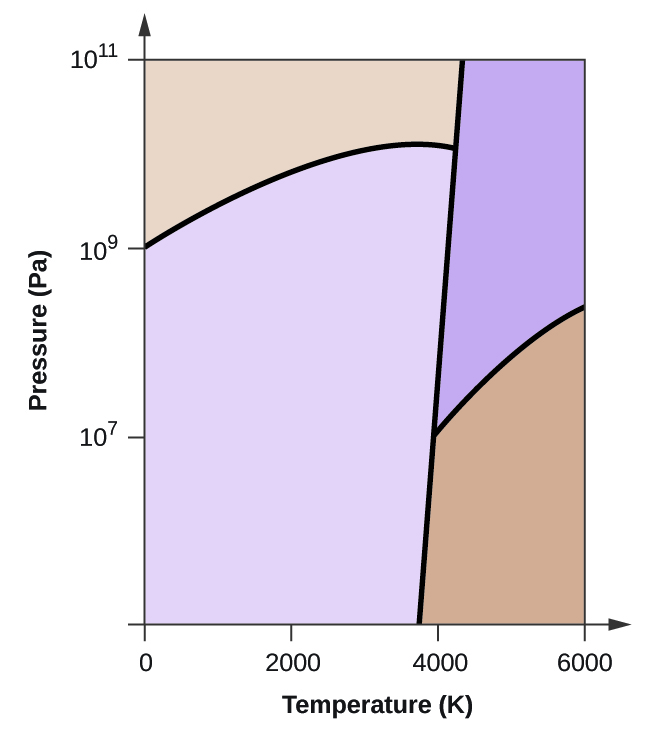
(a) On the phase diagram, label the gas and liquid regions.
(b) Graphite is the most stable phase of carbon at normal atmospheric condition. On the phase diagram, characterization the graphite phase.
(c) If graphite at normal conditions is heated to 2500 Grand while the pressure is increased to 1010 Pa, information technology is converted into diamond. Label the diamond phase.
(d) Circle each triple point on the phase diagram.
(e) In what stage does carbon exist at 5000 Thou and 108 Pa?
(f) If the temperature of a sample of carbon increases from 3000 M to 5000 K at a abiding pressure of 106 Pa, which stage transition occurs, if any?
(a)
(b)
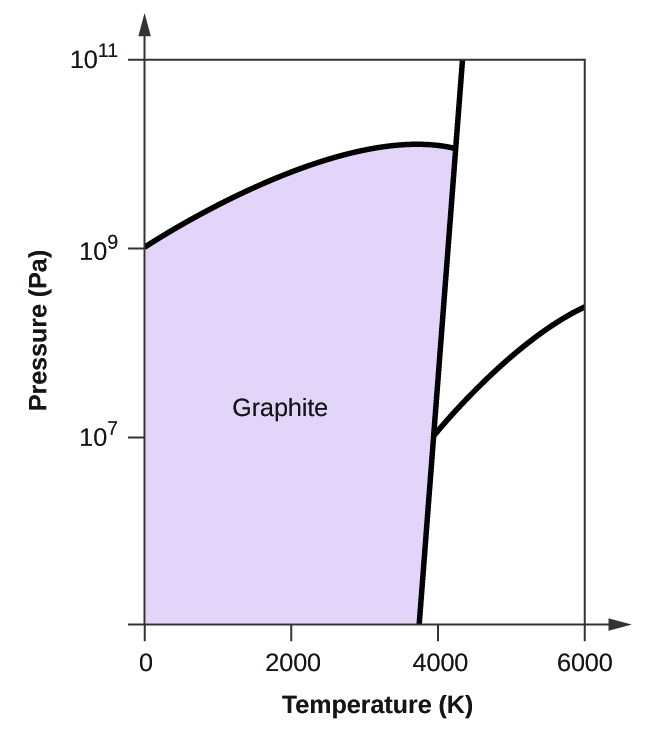
(c)
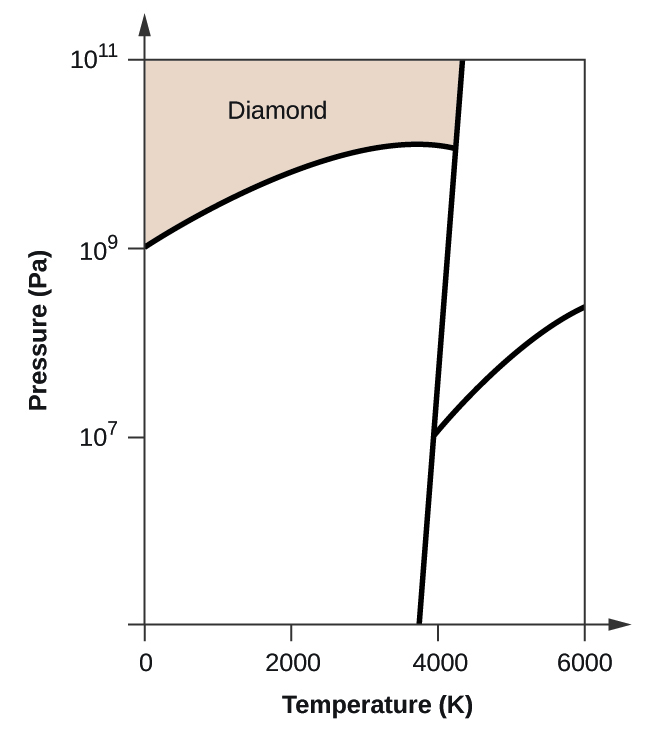
(d)
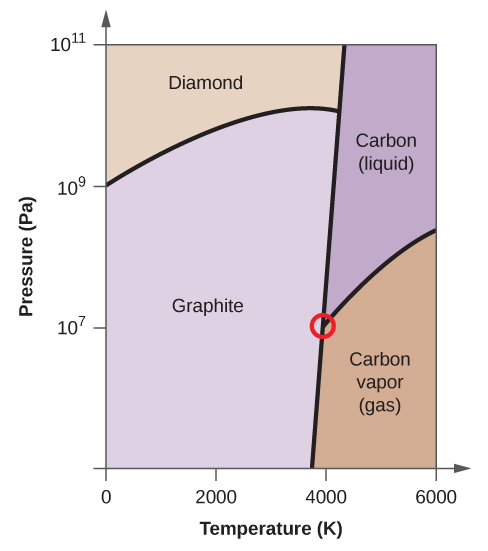
(due east) liquid phase (f) sublimation
Glossary
- critical point
- temperature and pressure above which a gas cannot be condensed into a liquid
- phase diagram
- force per unit area-temperature graph summarizing conditions nether which the phases of a substance can exist
- supercritical fluid
- substance at a temperature and pressure higher than its critical point; exhibits properties intermediate between those of gaseous and liquid states
- triple point
- temperature and pressure at which the vapor, liquid, and solid phases of a substance are in equilibrium
littlefieldmonce1990.blogspot.com
Source: https://pressbooks-dev.oer.hawaii.edu/chemistry/chapter/phase-diagrams/
0 Response to "what needs to happen in order for cession in hawaii to occur?"
Post a Comment